Results enhance existing evidence base showing Spiolto Respimat improves symptom reduction and quality of life over Spiriva Respimat2,3,4,5,6
For media excluding the United States of America, Canada and the United Kingdom
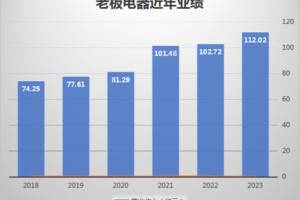
INGELHEIM, Germany–(BUSINESS WIRE)–Boehringer Ingelheim today announced data from the landmark 52-week DYNAGITO® trial which show that in people with COPD (chronic obstructive pulmonary disease), Spiolto® Respimat® (tiotropium/olodaterol 5/5µg) lowers the rate of moderate-to-severe exacerbations compared with Spiriva® Respimat® (tiotropium). The pre-specified significance level of p<0.01 for the primary endpoint of DYNAGITO® was not met.1 Treatment with tiotropium/olodaterol resulted in a 7% lower rate of moderate-to-severe COPD exacerbations compared with tiotropium alone (p=0.0498).1 This study, involving more than 7,800 people with COPD over 1 year, was published today in The Lancet Respiratory Medicine.1
“The results of DYNAGITO® are of value, as they show that tiotropium/olodaterol can lower the rate of moderate-to-severe exacerbations in many patients compared to tiotropium – a tough comparator which has consistently demonstrated exacerbation risk reduction through long-term, real-world experience,” said study investigator Professor Peter M.A. Calverley, Professor of Pulmonary Medicine, University of Liverpool, UK. “These data support evidence-based expert recommendations that dual bronchodilator LAMA/LABA therapy has a central role in the management of people with COPD, in terms of symptom improvement and exacerbation risk reduction.”7
COPD is a progressive, yet treatable condition that significantly impacts patients’ lives, restricting their daily activities from early on in the disease.8,9,10 COPD exacerbations, or flare-ups, are sudden episodes of increased breathlessness, cough and mucus production that can last for several days or even weeks.11These episodes can be seriously disabling, resulting in a need for urgent medical care, including hospitalisation, and sometimes lead to death.11
Further DYNAGITO® data demonstrated that tiotropium/olodaterol was associated with fewer moderate-to-severe exacerbations that needed intervention with a systemic corticosteroid, with or without antibiotics, compared with tiotropium:1*
- A 20% lower rate of moderate-to-severe exacerbations that required treatment with a systemic corticosteroid (p=0.0068).1*
- A 9% lower rate of exacerbations where the use of both a systemic corticosteroid and antibiotics were needed (p=0.0447).1*
- No difference was observed in the rate of exacerbations that required treatment with antibiotics only (p=0.2062).1*
No new side effects or safety concerns were identified in the DYNAGITO® study.1 These data also show that tiotropium/olodaterol has a similar safety profile to tiotropium.1
Reducing symptoms and the future risk of exacerbations are key treatment goals for COPD.7 According to the international GOLD† 2018 Strategy recommendations, LAMA/LABA treatments such as tiotropium/olodaterol play a central role in the management of COPD and help to achieve these treatment goals.7
Intended audiences:
This press release is issued from our Corporate Headquarters in Ingelheim, Germany and is intended to provide information about our global business. Please be aware that information relating to the approval status and labels of approved products may vary from country to country, and a country-specific press release on this topic may have been issued in the countries where we do business.
For references and notes to editors, please visit: http://www.boehringer-ingelheim.com/press-release/results-landmark-dynagito-trial
* The pre-specified significance level of p<0.01 for the primary endpoint of DYNAGITO® was not met
† Global Initiative for Chronic Obstructive Lung Disease
Contacts
Boehringer Ingelheim
Corporate Communications
Media + PR
Dr. Carolin Grob
Email: carolin.grob@boehringer-ingelheim.com
Phone: +49 (6132) 77-182603
Fax: +49 (6132) 77-6601
or
Email: press@boehringer-ingelheim.com
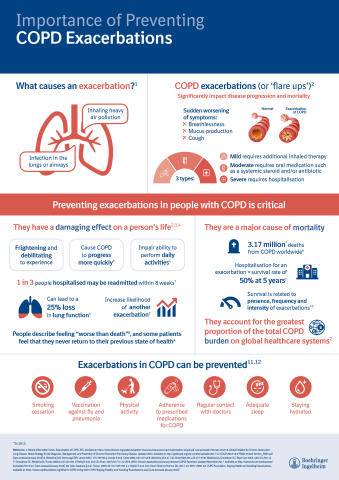
Importance of Preventing COPD Exacerbations (Infographic: Business Wire)

DYNAGITO One of the Largest Studies to Date of Exacerbations in COPD (Infographic: Business Wire)
✽本文资讯仅供参考,并不构成投资或采购等决策建议(BW)。